 |
| SITN presenters: Matt Davis, Tim Webster, and Daniel Field. |
During last week's Science in the News, Matt, Dan, and Tim described to us some of Earth's prehistoric inhabitants, and gave us a window into the history of the world before Homo sapiens. We learned that Torosaurus appeared 70 million years ago, and that the first members of our genus, Homo, walked the earth 2.4 million years ago. Then someone asked a fantastic question: How did we figure out all of these dates?
Using this Torosaurus skull, let’s figure out how exactly we determine the age of fossils.
To start off, this skull is made out of a huge number of atoms. The atom is the tiny component that makes up all matter on Earth. All atoms are composed of protons (red) and neutrons (green) in the center, or the nucleus, with electrons (yellow) orbiting around them.
Atoms with the same number of protons are grouped under the same element, such as carbon, oxygen, or nitrogen, and all atoms of the same element display similar chemical properties. But within each element, the atoms can also differ in the number of neutrons they have. These atoms are called isotopes, and are written with the sum of protons and neutrons after the element name.
-Natalie Ma First year BBS graduate student
To start off, this skull is made out of a huge number of atoms. The atom is the tiny component that makes up all matter on Earth. All atoms are composed of protons (red) and neutrons (green) in the center, or the nucleus, with electrons (yellow) orbiting around them.
 |
| Source: Wikimedia |
Carbon-12 | Carbon-13 | Carbon-14 | |
Number of protons | 6 | 6 | 6 |
Number of neutrons | 6 | 7 | 8 |
Protons + neutrons | 12 | 13 | 14 |
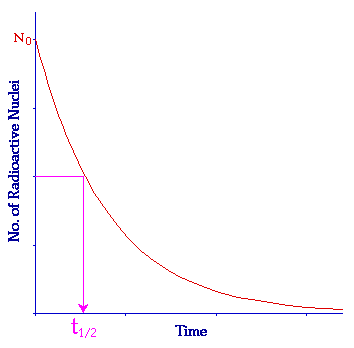
While most isotopes display identical chemical properties, a few are radioactive, which means they spontaneously decay into atoms of a different element while releasing radiation. The rate of this radioactive decay is constant and predictable, giving rise to what we call the half-life of an isotope, or the time it takes for half of all the original atoms in a sample to decay.
 |
| Source: Wikimedia |
So, if we’re looking at our Torosaurus skull, and we know it starts out with 1000 atoms of carbon-14, which has a half-life of 5,730 years, we know that in 5,730 years, 500 of the 1000 carbon-14 atoms will still be there. In another 5,730 years, or 11,460 years total, only 250 carbon-14 atoms will be left.
Radioactive isotopes (radioisotopes) occur in fossils and all other matter at low concentrations. In theory, we could then just measure the concentration of radioisotopes in our Torosaurus skull and be done with it, but it’s not that simple. The problem with directly measuring radioisotopes in the Torosaurus skull is that we don’t know how many of each radioisotope it started out with, and thus have no starting point for our clock. Fossils and the sedimentary layers they are found in are composed of rocks from all over the place that then squeezed together, so trying to date them with radioisotopes gets us many different dates.
We may not be able to directly measure the age of our Torosaurus skull or the sedimentary layer it was found in, but we can guess its age using igneous rocks. Igneous rocks are formed by volcanic activity and carry a unique signature of isotope concentrations. By examining newly formed igneous rock from recent volcanic eruptions, we can learn what concentrations of radioisotopes igneous rocks start out with and can "set" our clock. We can then figure out the age of the igneous rock formations nearest to our fossil, and give an age range for how old our Torosaurus skull is.
Now we can say that our Torosaurus skull is between 269 million and 264 million years old. Once we’ve figured this out, we can look at the unique features of this sedimentary layer, such as the presence of Torosaurus, and use them to identify the same sedimentary layer at another site, even though the layer may not have the same igneous rock formations above or below it at this site. Using this method, we can estimate the age of any fossil discovered!
There is one isotope that can be used to directly tell the age of a recent fossil. Carbon-14, the radioisotope of carbon, builds up a unique signature in plant and animal tissue because plants absorb carbon-14 from the atmosphere, where it is continually formed by incoming cosmic rays. Because animals either eat plants directly or eat other animals that consume the plants, they also gain the same carbon-14 signature in their tissue. When an organism dies, carbon-14 continues to decay at the constant rate of its half-life but no new atoms are added. As a result the concentration of carbon-14 in the organism's matter returns to zero over time, and measurements of carbon-14 can tell us directly how old a fossil is. Unfortunately, carbon-14 has a very short half-life (5,730 years), so if we tried to measure the amount of carbon-14 in our 264-269 million-year-old Torosaurus skull, it would’ve been zero. Carbon-14 measurements can only be taken on relatively young samples, such as those found at archaeological sites.
There are a few different isotopes commonly used in radiometric dating, so I've compiled a list of their properties below. To increase accuracy, researchers often measure both the amount of the listed isotope and the amount of the isotope it decays into (decay product). Which ones do you think we could use to determine the age of our Torosaurus skull?
Isotope | Decay Product | Half-life | Range it can predict accurately |
Carbon-14 | Nitrogen-14 | 5,730 years | 58,000 to 62,000 years |
Potassium-40 | Argon-40 | 1.25 billion years | 100,000 years to 4.5 billion years or more |
Uranium-238 | Lead-206 | 4.5 billion years | 1 million to 4.5 billion years or more |
Thorium-232 | Lead-208 | 14.0 billion years | 1 million to 4.5 billion years or more |
Rubidium-87 | Strontium-87 | 48.8 billion years | 1 million to 4.5 billion years or more |
Samarium-147 | Neodymium-143 | 106 billion years | 1 million to 4.5 billion years or more* |
*this is used to measure the age of meteorites and other cosmic material
Sources:



No comments:
Post a Comment